The state of Ohio has, as of this writing, five confirmed cases of Covid-19, the disease caused by the coronavirus SARS-CoV-2. If you ask Amy Acton, the director of the Ohio Department of Health, that’s the tip of the tip of a very dangerous iceberg. “We know now, just the fact of community spread says that at least 1 percent, at the very least 1 percent, of our population is carrying this virus in Ohio today," Acton said at a press conference on Thursday. "We have 11.7 million people." In other words, Acton was implying: 117,000 cases in Ohio alone.
How could Acton know this? Well, technically, she can’t. She was extrapolating from what little data scientists actually have about the spread of the pandemic. Officially, there have been just shy of 130,000 cases of Covid-19 on Earth, so a six-digit toll among Ohioans would be terrible, with extraordinary implications for how many cases might be out there undetected in the rest of the country.
Whether that number is accurate or not is exactly the sort of thing public health workers would like to know. Who’s sick? How bad? How fast will the disease spread? But in the United States, nobody has good answers, for one simple but terrifying reason: There aren’t enough tests. Unlike for the flu, or previous coronaviruses like SARS or MERS, or sexually transmitted diseases, or a host of other infections, health workers have no way to find out whether a person sitting in front of them has Covid-19 or not. That technology actually exists and is relatively simple. They just don’t have access to it. Right now, four months into a global pandemic, two weeks since the first case of “community spread” within US borders, doctors and health workers have no way of testing everyone to see whether they have Covid-19. (That may at last be about to change. On March 13, the FDA granted approval for commercial tests from two companies, Swiss pharmaceutical giant Roche and medical device-maker Thermo Fisher. Their expertise in fast, bulk-scale medical test construction and distribution means their promise to roll out 2 million tests almost immediately is actually plausible.)
The American Enterprise Institute’s program to estimate total US testing capacity currently puts that number at just over 22,000 tests a day—roughly twice the daily capacity of South Korea, a country with just one-sixth the population of the US and about one-hundredth the square mileage. That shortfall limits what scientists know about the disease—and therefore what they know about how to fight it. America is behind a fearsome curve, looking at a potential explosion of infection numbers. A large percentage of Americans will get sick enough to require hospitalization, and some of them are going to die. But nobody had enough tests in January or February to try to get ahead of that problem, and nobody has enough tests now to know how big a wave is yet to come. The reasons why are both scientific and political, and it’ll take deft science and politics to fix them.
Broadly, the response to an infectious disease outbreak has two phases: containment and mitigation. The containment phase happens early, when initial cases begin to appear. Public health workers tasked with surveillance do interviews to determine all the people an infected person might have come into contact with, to notify them and either isolate or treat them, in an effort to reduce further spread. But you can see where this is headed: Without the ability to test whether people are infected, health workers can’t effectively wall off these networks.
In the second phase, when the disease has spread beyond any public health system’s ability to investigate and handle individual cases, people need to understand what they’re up against. Without the ability to test vast numbers of people, it’s hard to calculate how many of the sick will need hospitalization, or will likely die. These numbers will be (figuratively and literally) all over the map. With Covid-19, the estimated percentage of infected people who die of the disease, or the “case fatality rate,” has varied from less than 1 percent to 15 percent, depending on the depth of testing conducted by each nation and how much data they actually released. Lots of factors might explain this variance, from a population’s overall wellness to its demographics to the nation’s health care system. It’d be nice to know which percentage is correct.
As the fight moves from individuals to entire populations, the goal of mitigation measures like social distancing, canceling large events, closing schools, and telling people to work from home is, sure, to lower the number of infections, but also to “flatten the curve,” as epidemiologists say. That curve is the number of sick people at a given time, and the best thing that could happen right now would be squishing that tsunami into a ripple, not necessarily reducing the overall number of infections but spacing them out, slowing them down. That’s because US hospitals have limited staff, equipment, and space, with just 2.9 hospital beds per 1,000 people. That’s fewer than Italy but more than Iran, whose health systems both got utterly slammed. And that’s also in the hope that, with more time to work the problem, new treatments might get figured out.
When testing for a new virus like SARS-Cov-2, the first wave of diagnostics almost always relies on two important (though not particularly modern) technologies.
The first, PCR, or polymerase chain reaction, is a DNA amplification technique that is routinely used in the lab to turn tiny amounts of DNA into large enough quantities that they can be analyzed. Invented in the 1980s by Kary Mullis, the Nobel Prize-winning technique uses cycles of heating and cooling to make millions of copies of a very small amount of DNA. When combined with a fluorescent dye that glows in the presence of DNA, PCR can actually tell scientists how much DNA there is. That’s useful for detecting when a pathogen is present, either circulating in a host’s body or left behind on surfaces.
But if scientists want to detect a virus like SARS-CoV-2, they first have to turn its genome, which is made of single-stranded RNA, into DNA. They do that with a handy enzyme called reverse-transcriptase. Combine the two techniques and you’ve got RT-PCR.
Currently, RT-PCR is the only way to determine if a person has Covid-19. No other kinds of tests can yet distinguish the virus that causes it from influenza or the other dozen or so respiratory bugs that are circulating this time of year. “It’s a very standard, reliable technique used in microbiology labs almost everywhere that can be quickly applied to clinical testing,” says Louis Mansky, director for the Institute of Molecular Virology at the University of Minnesota. “It’s the fastest possible kind of test to develop.”
But until other kinds of tests can be developed and approved, all Covid-19 tests have to be conducted in a lab by trained technicians. They require PCR machines and people trained to use them, so they can’t be performed in a clinic or inside a patient’s home. Since PCR is such a workhorse of the biology world, lots of research labs at universities and hospitals have the necessary equipment and personnel, says Manksy. But in the US, only labs that have been certified by the federal Centers for Medicare and Medicaid Services can process clinical samples. That approval process can take months. “We have one of the more cumbersome—or, you could say, diligent—regulatory systems for testing,” says Bruce Carlson, publisher of Kalorama Information, a market research firm that covers medical diagnostics. “We’re very concerned about false positives, just as damning as false negatives.”
The test itself only takes about a day to run if you have all the required reagents. But shortages and shipping logistics can easily add days or even weeks to get a result. (This is, in fact, already happening, but more on that later.) Let’s start with what should happen:
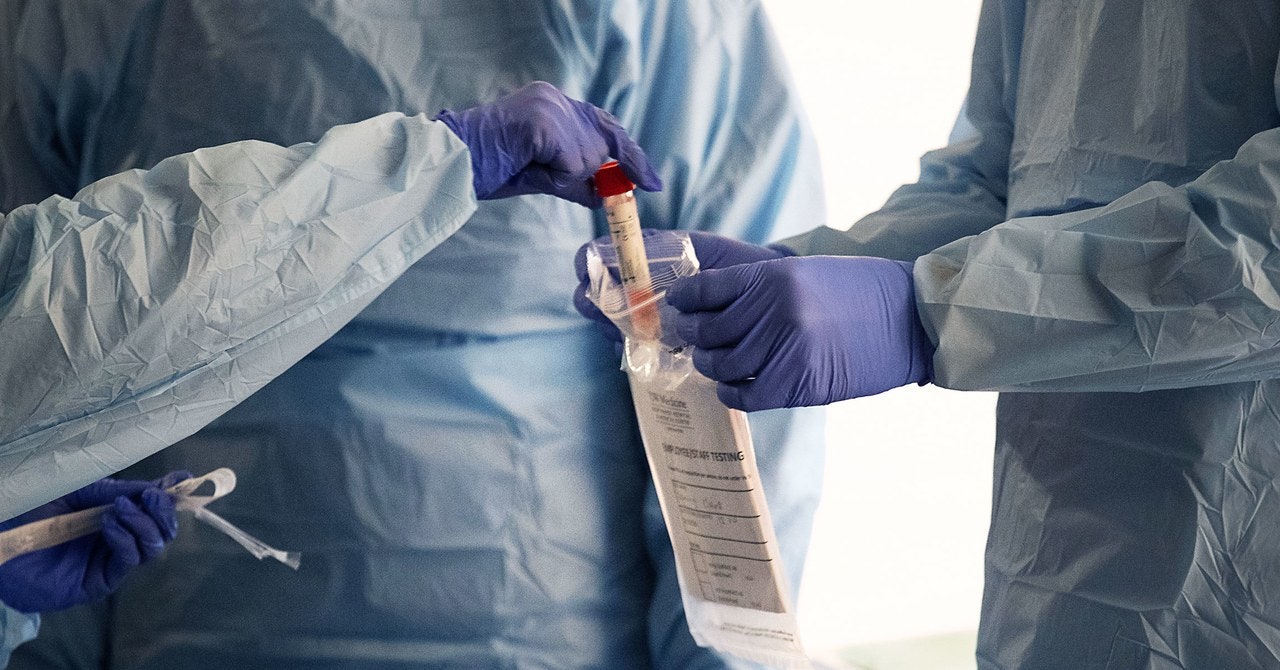
The very first step is collecting a sample. Using a sterile soft plastic stick, health care workers swab the inside of a patient’s nose or back of the throat. The goal is to collect material that’s recently been in the lungs, where the virus is believed to replicate. That stick then gets sealed up and shipped in a cold container to the testing lab. The sample has to stay between 35 and 40 degrees Fahrenheit, and if it’s not processed within four days it either goes into a freezer or gets thrown out.
Once in a lab, the first step is to separate out the RNA from everything else in the sample—human cells, proteins, enzymes that would chew up that viral genetic code. This is called RNA extraction. If you’re doing it by hand, this process usually involves adding chemicals and centrifuging the sample so the RNA winds up in a different layer from everything else. Several large biochemical suppliers make kits with everything you’d need to RNA extraction. There are also automated machines that do it, too.
Once the RNA has been purified, the next step is to add the reverse transcriptase enzyme that converts it to DNA—going from one strand to two. Then the DNA goes into a test tube along with batches of loose nucleotides, a DNA-building enzyme, and short synthesized DNA fragments called “primers.” These primers have been designed to find and bind to specific segments of the viral genome. In other words, they should, if they work right, recognize and amplify only genetic material from the virus, and not from anything else that might be in the sample, like human or bacterial DNA.
This all happens inside a PCR machine, an instrument that runs coordinated temperature cycles. As it heats the tube, the DNA’s double helix separates into two strands, exposing each side. When it subsequently lowers the temperature, the primers lock onto their targeted segments of the exposed DNA. The enzyme uses these primers as a starting place and begins building complementary strands of DNA according to the exposed sequence. About five minutes later, where once there was one strand of DNA, now there are two. After 30 to 40 cycles of this process, a single copy of DNA multiplies to hundreds of millions. That’s enough DNA that scientists can begin to detect it.
They do that with a fluorescent dye that is added to the test tube during the PCR amplification phase. It only glows in the presence of DNA. As the number of copies of DNA increases, so does the amount of light emitted. A special light-measuring instrument inside the PCR machine then reads out these fluorescence patterns to determine which samples have the virus in them and which don’t. “If there’s coronavirus in your sample, then its RNA will be transcribed into DNA and amplified along with a fluorescent signal that tells you if the test is positive or negative,” says Mansky.
What’s important to remember about RT-PCR is it’s not one test for one virus. It’s a method for identifying specific genetic sequences used in academic, commercial, and public health labs around the world. And the exact recipe scientists follow to get trusted results—which RNA extraction kit, which PCR machine, which primers—can vary. These recipes are referred to as “protocols.”
When a novel disease like Covid-19 emerges, universities, national research institutes, and public health organizations like the US Center for Disease Prevention and Control are usually the first to produce RT-PCR protocols. They have the biosafety labs to handle deadly new pathogens, including the ability to grow them—a crucial step for validating any tests. Once those agencies have a working test, they can deploy it to local public health labs and hospitals. Eventually, if the outbreak sticks around, commercial labs and diagnostic companies will produce their own tests, which may or may not require the same amount of expertise and manual lab work.
Starting in January, shortly after Chinese researchers released the first whole genome sequence of SARS-CoV-2, groups around the world began designing, testing, and publicly posting protocols for detecting the new coronavirus with RT-PCR. As a resource for testing labs, the World Health Organization has been keeping a list of these protocols, as well as guidelines for best practices.
Among them is a protocol developed by the US Centers for Disease Prevention and Control. Its test consists of four sets of primers. The first two, called N1 and N2, target unique regions of the SARS-CoV-2 genome that code for a protein that encapsulates and protects the virus’s genetic material. The third primer targets a gene common to the whole family of SARS-like viruses. The fourth and final primer targets a human gene, which serves as a positive quality control. Basically, it’s a target that the test should pick up 100 percent of the time. If it doesn’t, it’s a sign the test isn’t working the way it should. The kits also include instructions for testing a negative control—DNA that isn’t related to SARS-CoV-2—which should not react with the first three primers. The CDC began using this test in January on a limited number of samples from people with a history of travel to Wuhan, then the epicenter of the Covid-19 outbreak.
On February 4—two weeks after the CDC had detected the first Covid-19 case in the US—the US Food and Drug Administration issued emergency authorization of the CDC’s test, skirting normal regulatory channels to make it available to certified public health labs around the country. The CDC packaged these primers, along with their associated fluorescent probes, into kits that could each be used to process between 700 and 800 samples. Starting on February 5, the CDC finally began sending out 200 of those kits to the 115 domestic public health labs qualified to run the test through its distribution arm, the International Reagent Resource.
That’s where things started to go sideways.
As soon as the test kits arrived in the state laboratories, scientists there set about the first order of business—verifying the tests’ results. That involved running known samples through the test to make sure it picked up SARS-Cov-2 when it should, and didn’t erroneously flag harmless, virus-free samples. But a number of those labs ran into issues. On some, the negative control failed. On others, what should have been positive results came back as inconclusive.
On February 12, the CDC announced that the problem was the result of a faulty reagent. The third primer, the one that picks up the whole family of coronaviruses, wasn’t working properly. CDC officials told labs to sit tight, new kits were coming. As a result, for weeks, only a handful of laboratories in just a few states had the ability to test for Covid-19. Everywhere else, health departments with suspected cases on their hands had to send samples directly to the CDC for testing. And under the CDC’s narrow testing guidelines at the time, only people with symptoms and a history of travel to China were eligible to receive a test. This meant many infectious people were missed during the crucial early days of the virus’s spread to the US, as The New York Times reported.
As the CDC struggled to get out more tests, other countries experiencing their own outbreaks moved to adopt other protocols publicized by the WHO. And in the US, by February 25, only 12 US labs outside the CDC, in just five states, had the ability to test for the virus. At the time, only 426 people in the US had been tested, according to archived versions of the CDC website. (As of March 2, the agency stopped reporting the number of people tested.) Meanwhile, South Korea was reportedly testing 15,000 people per day—almost as many as the United States has tested to date in total.
In principle, any certified lab in the US with the right equipment could have followed suit—including hospitals and commercial labs. All they’d need to do is use any one of the protocols publicized by the WHO as a guide for ordering primers from any number of DNA synthesis companies and push forward with testing. But in reality, they were paralyzed, stuck behind a wall of freshly stretched red tape, able only to wait on the CDC to ship more kits.
See, when federal health authorities declared Covid-19 a public health emergency back in January, it triggered a set of rules requiring any tests to pass the FDA’s “emergency use approval” process. These rules raise the bar for tests developed and run inside a single laboratory. In a non-emergency situation, the FDA generally stays away from regulating these kinds of tests. But under the emergency rules, any lab that wanted to test for Covid-19 had to perform a number of validation studies and submit data to the FDA for review. These require viral specimens, which can be hard to obtain if you’re not the CDC.
For consistency’s sake, the FDA opted to limit its initial emergency approval to just the CDC test, to ensure accurate surveillance across state, county, and city health departments. “The testing strategy the government picked was very limited. Even if the tests had worked, they wouldn’t have had that much capacity for a while,” says Joshua Sharfstein, a health policy researcher at Johns Hopkins School of Public Health and the coauthor of a recent journal article on how this testing system has gone awry. “They basically were saying, we’re going to use a test not only developed by CDC, but CDC has to wrap it up and send it to the lab, and it’s just going to be state labs doing it.”
The effect was that the nation’s labs could only run tests using the CDC’s kits. They couldn’t order their own primers and probes, even if they were identical to the ones inside the CDC kits. And when the CDC’s kits turned out to be flawed, there was no plan B.
But no one outside the government yet knows why, at that moment, the US didn’t switch to a test developed for the WHO, in use in more than 120 countries. A spokesperson for the WHO declined to answer that question, and US policymakers like Health and Human Services secretary Alex Azar have repeatedly dodged it. It might have been straight-up bureaucracy—getting that test approved for use in the US might have taken too long. Some people suspect the answer may be politics. Speaking to the NPR program Fresh Air, Dan Diamond, health policy reporter for Politico, claimed that President Donald Trump had intentionally slow-walked testing deployment because higher numbers would jeopardize his reelection campaign.
On February 29, facing mounting pressure to expand testing capacity in the face of a growing public health catastrophe, the FDA changed its regulations to allow certified clinical labs to develop and begin using their own tests to detect Covid-19 without prior approval. “As soon as they found community transmission cases on the West Coast, that was a wake-up call,” Sharfstein says. “By the end of the week, the FDA just said, ‘Get started.’”
Under the new policy, the FDA review will still be required, but labs will have two weeks to send data to the FDA after internally validating the tests. In the meantime, they can start using their diagnostics to test patient samples.
With the relaxed protocols, two of the largest diagnostic commercial labs, Quest Diagnostics and LabCorp, have begun conducting a few thousands tests a day, according to data compiled by the American Enterprise Institute. (LabCorp representatives didn’t return a request for comment, but a spokesperson for Quest says the company expects to have the capacity to conduct “tens of thousands of tests per week” by the end of March.) Universities and hospitals have also booted up.
But where regulatory barriers have been removed, logistical ones have arisen. As more labs come online, nearly every step in the RT-PCR test has the potential to run into bottlenecks. The biggest one, right now, says Association of Public Health Laboratories chief program officer Eric Blank, is RNA extraction kits. “Everyone is trying to order these ancillary ingredients, and we’re hearing from our member labs that many of them are impossible to get right now.”
The CDC’s protocol recommends an RNA extraction kit sold by the company Qiagen. But as Politico first reported on March 10, those kits are now on backorder, due to the “extraordinary pace” at which the world has increased coronavirus testing over the last few weeks. There are also reports of automated RNA extraction machines and RT-PCR instruments being on back order. Not all labs have versions of these machines that have been cleared by the CDC as qualified for Covid-19 testing. The ones that don’t are now scrambling to source them.
Qiagen is hiring new staff and ramping up its manufacturing sites in Europe, increasing to three shifts a day, seven days a week, company officials told WIRED in an emailed statement. And the FDA has recently moved to allow test-makers to substitute kits from another European manufacturer, Roche. But the current shortages are expected to further hamper the US’ ability to test potential Covid-19 patients in a timely manner. “There’s not going to be a fast solution to this,” says Blank. “It could take several weeks to resolve this supply chain issue, meaning that the demand for testing is going to outstrip supply for the foreseeable future.”
More testing kits would certainly ramp up capacity. Eventually, new testing technologies might also help.
At least 20 companies have announced plans to develop “molecular point-of-care” tests, basically all-in-one, mostly automated systems that a frontline health care worker can use to get results in a half hour, instead of in days. “You take the sample from the patient, usually a nose swab or sputum, and then put it into a cartridge, put that into the machine, and hit a button. You get a diagnosis in 30 minutes, is what they’re aiming at,” says Carlson of Kalorama Information. “They can also run a high volume.”
Cepheid, one of the major companies that makes these kinds of kits for other diseases, has a Covid-19 version in the pipeline, and Coyote Bioscience already has one deployed in China. There’s even one in development that tests for both Covid-19 and influenza at the same time, good for excluding people from isolation wards and getting them appropriate care.
Crispr-based diagnostics for detecting the coronavirus are also in the works at startups like Sherlock Biosciences and Mammoth Biosciences. These tests use Crispr’s programmable gene-seeking capabilities to deliver a diagnosis in under an hour without the need for fussy lab instruments. Mammoth published a preprint on March 10, describing a test the company developed for SARS-CoV-2 that works with paper test strips like those you’d find in a drugstore pregnancy test. The company is currently in the process of further validating its initial results.
Another in-demand approach would look for antibodies to the virus in the blood of patients, a so-called serological test. That’d be useful, because in addition to identifying people with Covid-19, it could tell you if someone was once infected but then recovered. “The better your surveillance, the more cases you’re going to catch, but even with perfect surveillance you won’t catch everything,” says Martin Hibberd, an infectious disease researcher at the London School of Hygiene and Tropical Medicine who helped develop one of the first tests for the coronavirus SARS in the early 2000s. “Until we’ve got a full test of this type of assay, we don’t know how many cases we’ve missed.”
A serological test would also probably be cheaper than a PCR-based one, and more suited to automation and high-throughput testing. A researcher in Singapore is testing one now.
Serological tests may be less accurate than molecular ones—but on the plus side, Hibberd says, SARS-CoV-2 also shows up on the serological tests designed for the original SARS virus. Whether that’ll help with developing new tests is unclear. “I went back to my freezer in Singapore—that’s part of why I’m here—and all my samples were thrown away. People worried that the risk was higher having those samples than not having them,” Hibberd says. “All of the funding we had during the SARS period to develop the serology, to develop the vaccine, all disappeared as soon as the virus disappeared.”
This patchwork rollout of testing capacity means that whether or not you can get a diagnosis for Covid-19 if you feel sick depends largely on where you live. According to a recent report from The Wall Street Journal, some hospitals can’t provide testing, because they don’t have enough of the right kinds of masks to protect health care workers while they collect patient samples. Others are having to ration tests, limiting them to only the most severe cases, and encouraging people with mild symptoms to stay home. At this point, every state, county, and some cities have their own policies about who’s eligible to receive a test. “The system is not really geared to what we need right now,” Anthony Fauci, head of the National Institute of Allergy and Infectious Disease, told a congressional hearing this week. “That is a failing. It is a failing, let’s admit it.”
At a White House press conference on Friday, President Trump attempted to address that failing. He declared Covid-19 a national emergency, clearing the way for an infusion of funds and decreased regulation at the state and local level. The president also announced the creation of a public-private partnership with several national big-box stores and pharmacies, including Walmart and CVS, to allow parts of their parking lots to be set aside for drive-through testing. Partnerships with testing companies like Roche, Quest, and LabCorp meant that “we’ll have the ability to do in the millions over a very, very quick period of time,” the president said. Vice President Mike Pence said they expected to eventually spin up to between 15,000 and 20,000 tests a day.
There’ll be a new system for getting tested too. Pence said that two days after the press conference he’d release information on the timing of the debut of a new website, to be created by Google, that would allow people to enter their symptoms and, if they met certain criteria, get the location of a parking-lot drive-through test facility where they would be swabbed for SARS-CoV-2. The implication seemed to be that people still need to have symptoms to get tested, which doesn’t address some of the broader epidemiological questions. Google has since denied that it or its sibling health care company Verily is working on any such technology, other than a small triaging pilot project for health care workers that Verily is intending to test in the Bay Area.
The president also dismissed a question about his administration’s culpability in the lack of tests available so far. “I don’t take responsibility at all,” he said. “We were given a set of circumstances, and we were given rules, regulations, and specifications from a different time.” Reminded that he’d fired the people who would have been in charge of handling infectious disease outbreaks, the president said, “When you say ‘me,’ I didn’t do it. I don’t know anything about it.” What actually happened is a complicated series of bureaucratic dominoes, but it’s not true that Trump didn’t know about it.
The president’s press conference also left unanswered questions about helping people pay for testing and treatment, even though he’d conflated those two issues in a speech to the nation just two nights previous. (California, Washington, and New York have already told insurers they have to waive testing fees, and the new coronavirus aid bill negotiated by the House of Representatives and the Trump administration includes free testing.) It also wasn’t clear what would happen to people’s health data once they handed it over to the web site Google is supposed to be designing, nor what would happen next if they tested positive at a drive-through.
What should you do in the meantime? Practice your own social distancing, avoiding large gatherings. If you can work from home, it’s time to do that. If you learn you’ve had contact with someone who has Covid-19, self-isolate for 14 days. If you start to feel sick, do the same. Don’t go to the hospital if it’s the kind of mild illness that would normally just keep you home. (Hospitals are for people with chest pain, or who are having trouble breathing.) Wash your hands a lot, with soapy water, for at least 20 seconds. Maybe clean your phone and other gadgets. Stop touching your face, even though it’s hard. Check in on your neighbors, especially if they’re elderly or sick. They might need supplies.
And remember that this pandemic hurts everyone in different ways—the economic hit to store owners, the lost school time for kids, the potential for illness or worse. But it hurts the most vulnerable people the most. People who don’t have health insurance, who don’t have the kind of jobs that include sick leave, who don’t have ready access to child care, or who already have weakened immune systems from other issues are bearing the biggest load here. Until governments catch up to helping them out, anything you can do for them is going to be part of the real fight against the disease, the one we’re already in.
Updated 3-16-20 6:42 PM EST: This story was updated to include the FDA's approval of two commercial tests on March 13.
Updated 3-17-20 12:330 PM EST: This story was updated to correct the square mileage of South Korea.
WIRED is providing unlimited free access to stories about the coronavirus pandemic. Sign up for our Coronavirus Update to get the latest in your inbox.
- What's social distancing? (And other Covid-19 FAQs, answered)
- Don't go down a coronavirus anxiety spiral
- How to make your own hand sanitizer
- Singapore was ready for Covid-19—other countries, take note
- Is it ethical to order delivery during a pandemic?
- Read all of our coronavirus coverage here
Read more: https://www.wired.com/story/everything-you-need-to-know-about-coronavirus-testing/

Recent Comments